The Art Science and Technology of Pharmaceutical Compounding 3rd Edition
Advertizing

Compounding Preparations for Ophthalmic Utilize in Humans
INTRODUCTION
The human heart—an organ that is exposed to the ambience environment—theoretically should permit piece of cake access to administer medications. Actually, the center's construction resists administration of topical ophthalmic medications. Compounders, pharmacists, and pharmacy technicians need to be familiar with eye beefcake and ophthalmic preparations' components to choose and/or develop the best dosage class to care for various ocular disease states.
Eye Anatomy
The eye is essentially divided into 2 segments: anterior and posterior.i The heart'south anterior segment provides the organ'south first line of defence for preventing injury. Its primary barrier is the clear cornea and its iii layers: epithelium, stroma, and endothelium. The epithelium and endothelium layers are lipophilic and resist assimilation of aqueous or water-based substances.1 The epithelium, the cornea'due south outer layer, comprises ninety% of corneal cells. It is a tightly bound structure, which makes information technology very resistant to aqueous and hydrophilic substances. Although the endothelium, the inner layer, is also lipophilic, it is a unmarried layer of porous cells that allows molecules to pass into the eye'south anterior chamber.1
The stroma—the eye and thickest layer of the cornea—is hydrophilic. It is a highly organized network of collagen fibrils, which creates the center'south mechanical strength and transparency.ane The Bowmen'south membrane is also part of this hydrophilic layer and is located betwixt the epithelium and the stroma.2 The sclera or "white of the eye" is also hydrophilic and has permeability similar to the corneal stroma.
The posterior segment comprises the eye's back two-thirds and includes the vitreous sense of humor, retina, choroid, and optic nerve. These components are primarily responsible for vision.1
The eye's lacrimal system, another major component, produces tears. The lacrimal arrangement consists of lacrimal glands, lacrimal canals, a lacrimal sac, and a nasal lacrimal duct.ane Tears contain mucin, which forms a hydrophilic layer over the corneal surface. This layer lubricates the eye and flushes debris and pathogens. Tears have a pH of 7.four and are isotonic. The tear volume in humans is 7 mcL to nine mcL; however, the cul-de-sac or drainage canal of the upper and lower eyelids contains 20 mcL to thirty mcL of tears. The tear film's restoration fourth dimension is 2 to three minutes. Claret capillaries and lymphatics are located in the conjunctiva. Drugs tin be systemically absorbed in the conjunctiva, which significantly lowers the drugs' availability locally to the middle.1
OPHTHALMIC MEDICATION DRUG DELIVERY SYSTEMS
An ophthalmic drug delivery system'south goal is to achieve a therapeutic concentration of the agile drug in the target tissue for an appropriate duration. For example, an inflammatory condition in the eye's posterior segment probably cannot be treated effectively with a topical anti-inflammatory ophthalmic solution. It may require a more invasive treatment such as an intra-ocular injection. Nevertheless, patients or caregivers tin treat superficial corneal abrasions effectively with topical ophthalmic solutions or ointments. Selection of an ophthalmic dosage class is dependent on the centre's affected part, the drug'south chemic and physical properties, and patient compliance. Ideally, a medication drug delivery system for ophthalmic preparations should include the post-obit characteristics3:
- It should non induce a foreign-body sensation or long-lasting burning.
- Information technology should not crusade blurred vision for extended periods of time.
- It should human activity locally rather than systemically.
- Information technology should exist easy to administer.
- Administration frequency should be kept to a minimum to improve patient compliance.
Topical Dosage Forms
Topical ophthalmic dosage forms (solutions, suspensions, and ointments) are the nigh commonly prepared and used dosage forms for the eye. Patients or caregivers can administer them easily and they treat most diseases or conditions of the external eye or anterior segment finer. Since very little drug reaches the vitreous humor, these dosage forms are poor options for treating diseases affecting the centre's posterior segment (eastward.g., macular degeneration). The drug's effectiveness and ability to reach its target tissue site depends uponthree:
- The condition of the cornea and anatomical barriers of the eye
- The pharmacodynamics of the tear picture
- The physical properties of the drug and its vehicle
- The drug's bioavailability
There are 2 types of topical ophthalmic liquids: aqueous and nonaqueous. Aqueous solutions are quickly captivated and touch the patient'southward vision minimally. Aqueous solutions also do not interfere with practitioners' instruments during examinations or procedures. They take brief therapeutic effect and are good dosage forms for diagnostic procedures; nonetheless, they are poor treatment choices for chronic weather condition. Medications in aqueous vehicles, such as antibiotics, require frequent assistants to be effective. Almost topical solutions are washed away by tears within xv to xxx seconds of administration; less than 5% of the dose actually reaches the heart'due south posterior segment.4 Aqueous ophthalmic suspensions may be used to prolong drug effects. The active drug, located in the particles, slowly dissolves and releases over time. Topical aqueous ophthalmic liquids' disadvantage is their systemic assimilation by the alimentary tract later the liquid drains through the nasolacrimal duct.3
An reward of nonaqueous or "oily" ophthalmic solutions is that they form a film over the eye, allowing longer drug contact.i The film reduces drainage and decreases risk of systemic drug toxicity. The film also acts as an emollient and keeps the heart moist, which helps irritated and dry eyes. An oil-based solution, which lacks water, can increase the stability of a drug that is easily degraded past hydrolysis. Nonaqueous solutions' disadvantages include blurred vision and interference with practitioners' instruments.5
Topical ophthalmic ointments as well incorporate no water; they are adept vehicles for drugs that are unstable in aqueous solutions and undergo rapid hydrolysis.4 Adding an active drug to an ophthalmic ointment base offers multiple advantages. Information technology prolongs contact of the drug with the centre; provides tiresome, continuous absorption; and requires less frequent administration than aqueous topical solutions. These dosage forms are safer for drugs that have narrow therapeutic windows (e.g., atropine). Ophthalmic ointments are often administered at bedtime to minimize interference with the patient's vision. Topical ophthalmic ointments offer the same advantages every bit nonaqueous solutions and care for chronic conditions well. The disadvantages are also the same, such every bit blurred vision.4
Ophthalmic ointments are also prepared without any active drugs. They are used in trauma patients as a nonirritating, protective agent. These bland ophthalmic ointments also have no adverse upshot on corneal wound healing.6
Drugs commonly used in topical ophthalmic dosage forms include antibiotics, antifungals, anesthetics, steroids, dyes, and agents to care for chemical burns. Antibiotics and antifungals are used to treat astute superficial middle infections. Calcium gluconate and ascorbic acrid are used to care for chemical burns to the cornea.7,8 Cocaine is compounded off-label as an ophthalmic solution for anesthesia. Hyaluronidase ophthalmic solution is used off-label every bit an adjuvant for retrobulbar/peribulbar block.9,10
Cyclosporine is compounded in corn oil as a 2% nonaqueous solution and used off-label to prevent corneal immune graft rejection when used with topical ophthalmic steroid solutions. It is also used to treat vernal keratoconjunctivitis (KCS) or chronic graft-versus-host disease with KCS .11,12 The commercial product, Restasis or cyclosporine ophthalmic emulsion 0.05%, has an oily emulsion base which serves 2 purposes: it is an emollient and it keeps the active drug in contact with the eye to assist reduce inflammation due to chronic dry centre.xiii
Erythromycin ophthalmic ointment is administered to newborns at nascency to prevent ophthalmia neonatorum (contracted during passage through the birth culvert from a mother infected with either Neisseria gonorrhoeae or Chlamydia trachomatis).fourteen Rose bengal is compounded into a ane% solution as a diagnostic help for practitioners to diagnose various ocular injuries.15
Ocular Injections
Although ocular injections are invasive, uncomfortable, and inconvenient for patients, they may exist indicated to care for inflammatory atmospheric condition of the center, severe infections, macular degeneration, and to provide prophylaxis and anesthesia for ophthalmic surgical procedures. Ocular injections tin provide a therapeutic drug dose for an extended menstruation of time and may be preferred for ophthalmic atmospheric condition involving the eye's posterior segment. Different types of ocular injections target dissimilar eye areas: conjunctival, intravitreal, peribulbar, retrobulbar, subconjunctival, intracameral, and sub-Tenon'south.sixteen The common ocular and periocular injections are intravitreal and subconjunctival. Figure i illustrates the assistants of sub-Tenon'southward and intravitreal injections.
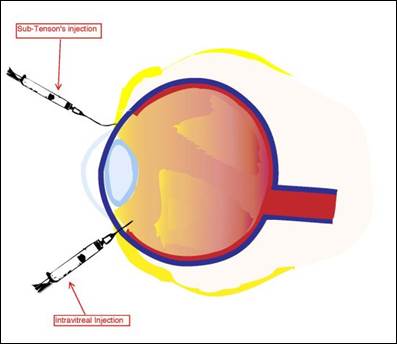
Figure 1. Administration of sub-Tenon's and Intravitreal Injections.
Diagram from the National Eye Found, Reference 2.
Periocular injections, such as subconjunctival and sub-Tenon's or episcleral space, are used to bypass the eye's anterior segment'due south concrete barriers. Subconjunctival injections are injected underneath the conjunctiva, while sub-Tenon'south injections are administered in the cavity between the Tenon's capsule and the sclera.17,18 These injections are typically used to administer antibiotics, corticosteroids, and mydriatics later on a surgical process or in acute inflammatory conditions of the anterior segment. Although non the outset line of therapy, subconjunctival injections are used to administer steroids in treating recalcitrant inductive and posterior uveitis. Subconjunctival injections deliver steroids to the eye steadily because the lipid-soluble steroid moves from the conjunctiva to the tear film where information technology is captivated through the cornea. Both the subconjunctival and sub-Tenon'southward injection have similar dosing volumes, about 0.v mL. Although periocular injections tin can be very effective, potential complications from administration include subconjunctival hemorrhage, pain, irritation, induced intraocular pressure spikes with steroids, secondary infections, chemosis, ecchymosis, perforation of the eye earth, and retained drug deposits.xix
Intraocular injections are administered directly into the centre's world and posterior segment to ensure the drug reaches the target site. Intracameral injections are injected into the center's aqueous humor, and intravitreal injections are injected into the centre's vitreous humor. Both injection types treat severe infections and emergent conditions, such as endophthalmitis. Due to the dosage forms' invasiveness, these injections are administered in a sterile clinical setting or operating room under local anesthesia. Dosing volume is unremarkably 0.i mL.17,18
The utilise of intravitreal injections has significantly increased since the 1970s and offer therapeutic advantages similar to periocular injections.16 Complications may include intraocular hemorrhage, hurting, irritation, induced intraocular pressure spike, secondary infection, optic nerve damage, retinal detachment, and uveitis or iritis. Drugs commonly administered intravitreally include antibiotics and antivirals, antiinflammatories, antineoplastics, and endothelial growth cistron inhibitors. These drugs are therapy's mainstay for ophthalmology and are used to treat endophthalmitis, viral retinitis, age-related macular degeneration, diabetic retinopathy, uveitis, vascular occlusions, and retinal disengagement.20,21
COMPONENTS IN OPHTHALMIC PREPARATIONS
Components or ingredients other than the active pharmaceutical ingredient and vehicle used in ophthalmic preparations or products are necessary to make stable, effective, and comfy ophthalmic dosage forms.22,23 Several factors must exist considered when compounding ophthalmic preparations: chemic stability of the active drug(south), possible microbial contagion, incompatibilities, viscosity, pH and buffering, tonicity, particle size (if a pause), concluding container (such every bit dropper bottle or syringe), compatibility with the eyes, appropriateness of the vehicle, and patient condolement and tolerability. Each component or ingredient should be assessed for compatibility with other components or ingredients in the ophthalmic preparation. The Handbook of Pharmaceutical Excipients contains detailed information about raw ingredients mentioned in the above sections, which should be consulted for a more detailed review.23
Preservatives
If possible, all multidose containers should comprise a preservative to foreclose microbial contagion during use.21 Preservatives often used in ophthalmic preparations and commercial products include benzalkonium chloride (BAK), chlorobutanol, benzethonium chloride, phenylmercuric nitrate, phenylmercuric acetate, and thimerosal. Table one lists these mutual preservatives and the usual effective concentrations.21 BAK and thimerosal are the about commonly used preservatives for ophthalmic preparations and products. BAK has a broad range of activity confronting a diverseness of bacteria, yeast, and fungi, but works the best against gram-positive leaner. Solutions with BAK remain stable over a wide range of pHs and temperatures without losing anti-microbial effectiveness. Some patients do, however, develop an allergy to BAK and may need their ophthalmic medication prepared without it. Soft contact lens wearers should avert solutions with BAK because it binds to the hydrogel.
| Table 1. Preservatives Used in Ophthalmic Preparations and Usual Concentrations | |
| Preservative | Usual Concentration (% w/v) |
| Benzalkonium Chloride | 0.013 |
| Benzethonium Chloride | 0.01 |
| Chlorobutanol | 0.25 to 0.five |
| Phenylmercuric acetate | 0.004 |
| Phenylmercuric nitrate | 0.004 |
| Thimerosal | 0.01 |
| Abbreviation: w/5 = weight to book Source: Reference 21 | |
Thimerosal is the alternative preservative to BAK and can be used by soft contact lens wearers. It is bacteriostatic and fungistatic at neutral and alkaline pHs, merely bactericidal at acidic pH levels. Although it is stable at room temperature, information technology is also lite sensitive.21
All preservatives have drug incompatibilities and a compounder must ensure the chosen preservative is uniform with all components in the ophthalmic dosage form. All ocular injections must exist prepared without preservatives, which tin can be toxic to the centre'southward internal structures, particularly when injected.24,25
Antioxidants
Antioxidants are added to ophthalmic preparations when the agile drug is susceptible to oxidation or degradation by gratis radicals. In other words, antioxidants stabilize the drug. Common antioxidants include ethylenediaminetetraacetic acid (more than usually known every bit edetate disodium [EDTA]), sodium metabisulfite, and sodium bisulfite. EDTA can too exist used equally an adjunct with BAK to heighten BAK's antimicrobial activity. Sodium metabisulfite is useful in acidic solutions, whereas sodium bisulfite is used in neutral pH solutions.22
Viscosity Agents
Viscosity agents thicken ophthalmic liquid vehicles, especially aqueous solutions, to increase contact time of the drug with the eye and minimize drainage into the nasolarimal system. Viscosity increases drug absorption and therapeutic effects. The viscosity of ophthalmic solutions ranges from 25 centipoise (cps) to fifty cps. Viscosity agents must commonly be sterilized by autoclaving before addition to an ophthalmic preparation considering they cannot exist sterilized through filtration. Usually used viscosity agents include hydroxyethylcellulose, hydroxypropyl methylcellulose (HPMC) or hypromellose, methylcellulose, polyvinyl alcohol (PVA), and polyvinyl pyrrolidione or povidone. PVA is commonly used in bogus tears and contact lens solutions, and is stable at temperatures beneath 100°C. HPMC is preferred over methylcellulose because it produces solutions with greater clarity and has soothing and lubricating properties.26
Tonicity Agents
Since human being tears are isotonic and very similar to 0.9% sodium chloride solution, information technology was idea that tonicity was important for ophthalmic preparations. Nonetheless, the eye tolerates tonicity ranging from 0.six% to ane.eight%. From a practical viewpoint, patients generally tolerate hypertonic solutions very well. Although the ideal osmolality value is 300 mOsm/50, nigh patients can tolerate an osmolality range of 200 mOsm/L to 600 mOsm/50. Only vehicles and lubricating solutions, such every bit bogus tears, must be isotonic. Common tonicity agents include dextrose, glycerin, and sodium chloride. Occasionally, a patient cannot tolerate a hypertonic solution because the active ingredient's sodium value is too high. If information technology is impossible to make the solution within an acceptable tonicity range, a viscosity agent may reduce some of the patient's pain and discomfort.26
Clarifying Agents
All ophthalmic solutions should be free and clear of any particulate affair to prevent abrasions to the cornea or eyelid. Filtering solutions through a 0.4 micron filter should remove all particulate matter without removing the drug. Using HPMC can serve a dual purpose in ophthalmic preparations. Not only is it a viscosity agent, merely HPMC can better the ophthalmic solution'south clarity. Polysorbates, e.g., polysorbate twenty and polysorbate 80, can as well amend clarity because these agents act equally solubilizing agents to assistance dissolve poorly soluble ingredients.26
pH and Buffering Agents
The pH can affect the chemic stability, potency, and effectiveness of drugs and components in a dosage form. An optimum pH avoids adverse furnishings, ensures that drugs will produce an optimum therapeutic issue, and ensures all components' roles are optimized. For example, most drugs are acidic or neutral and will precipitate, rendering the drugs inactive if added to a bones solution or vehicle. A precipitate in an ophthalmic preparation could also potentially crusade an corneal chafe. Some preservatives' antimicrobial action may be decreased if the pH is incompatible with the preservative. Every bit mentioned previously, thimerosal is bacteriostatic in neutral or basic pHs, but bactericidal in acidic pHs.
Buffers are used in ophthalmics when the pH is critical and must be inside a sure range. Buffering agents are designed to maintain a sure pH throughout the preparation'south shelf life. The system'south buffer capacity allows the tears' buffer organisation to bring the administered solution back to the tears' pH. Ideally the buffer capacity should exist less than 0.05 and maintain a pH range of 4 to viii. Ophthalmic buffering agents are ordinarily citrate, phosphate, or acetate buffers.26
Vehicles and Bases
Most vehicles and bases used in compounded ophthalmic preparations are commercially available, isotonic mixtures and may contain preservatives. Depending on the dosage form and indication, the vehicle or base may comprise lubricants, wetting agents or demulcents, electrolytes, viscosity agents, or buffering systems. It is very of import when preparing compounded ophthalmic medications to ensure that the vehicle or base is compatible with all of the formula's agile pharmaceutical ingredients and components. At that place are basically two types of vehicles and bases: aqueous and oleaginous or nonaqueous.22
Aqueous vehicles and bases are primarily used for topical solutions and ocular injections. It is very common to use commercial counterbalanced salt solutions or bogus tears as a vehicle for topical solutions; even so, different brands differ in content and may not exist interchangeable. Dextrose 5% in water for injection has been used every bit a vehicle for vancomycin l mg/mL ophthalmic solution and reported by patients equally comfortable when administered.27 If a formula for an ophthalmic preparation is based on a published stability study, the compounder must replicate the study formula exactly (i.e., utilise exactly the same brands or generics the study used) if they program to assign the beyond-employ dating (BUD) established in the written report. For example, if a stability study indicates information technology used the Systane brand of artificial tears, compounders cannot substitute some other make, like Murine Tears, and assign the BUD established in the study. Systane contains polyethylene glycol 400 and propylene glycol, whereas Murine Tears contains polyvinyl alcohol and povidone. These brands are not equivalent or interchangeable even though they are both artificial tear solutions. Prior to mixing and assigning a BUD, compounders must review the vehicles' contents to determine the compatibility of the active ingredients and components in a compounded ophthalmic formula.22
Ocular injections must exist prepared with preservative-complimentary diluents, such as 0.9% sodium chloride solution for injection, sterile water for injection, or five% dextrose solution for injection. These solutions may also be used to prepare topical solutions.26
Oleaginous vehicles and bases are primarily used to gear up ophthalmic topical ointments or topical "oily" solutions. When commercial ointments are used, all of the ophthalmic formulation'southward components need to accept been individually sterilized before mixing since an ointment cannot be filtered through a 0.22 micron filter. Nonsterile ointment bases tin can only be sterilized by dry heat. Autoclaves sterilize by a combination of estrus and pressure to create steam. Since an ointment contains no h2o, no steam is generated to sterilize it. Bland lubricating ophthalmic ointments that do not have active ingredients consist of three bones ingredients in various concentrations: white petrolatum, mineral oil, and lanolin.28 All of these ingredients are nonirritating and safe for use in the heart. Yellow petrolatum is not used in ophthalmic preparations because information technology is less purified than the white petrolatum and can be more irritating to the patient. For topical "oily" solutions, some fixed oils, such as corn oil or medium chain triglycerides oil, are too rubber to use and can exist sterilized by filtration through a 0.22 micron filter or with dry-oestrus.28
Active Pharmaceutical Ingredients
Ophthalmic drugs are used to prevent or care for eye diseases, salve uncomfortable symptoms that a patient may feel, or to assistance practitioners in ophthalmic diagnostic procedures. Active pharmaceutical ingredients (APIs) may exist obtained from commercial sterile products, such every bit parenterals, nonsterile bulk powders, or liquids. Most compounded ophthalmics can be prepared with commercial sterile products; yet, some compounders may need to prepare them using not-sterile components, which requires sterilizing processes and may crave extensive cease-training testing depending on the BUD and volume produced.22
COMPOUNDING OPHTHALMIC PREPARATIONS
According to the USP <797> chapter on sterile compounding, all ophthalmics, topical and injectable, are sterile preparations.24 At that place is a myth that ophthalmic preparations can be prepared in a not-sterile environs because they are no longer "sterile" one time the patient opens the container and administers the first dose. Even though at that place accept been numerous reports of microbial contamination of compounded ophthalmic medications resulting in injury, infection, or even loss of an center, some practitioners still prepare them in nonsterile conditions, such equally countertops in pharmacies, medication rooms, or at the patient's bedside.29-31 Ophthalmic preparations are often used to treat acute conditions and may be needed quickly. They are non considered emergent, life-threatening treatments, nevertheless, and should be prepared in a controlled, sterile environment. According to the proposed USP <800> affiliate on hazardous compounding, if the drug or components in the compounded ophthalmic preparation are hazardous, the compounder may need to prepare it in a negative-pressure level, ISO-7 room in a ISO-5 Class II biological safety cabinet or compounding aseptic containment isolator.32
Most compounded ophthalmic preparations are prepared using commercial sterile ingredients and usually are topical solutions or ocular injections. Notwithstanding, some compounded ophthalmic dosage forms are prepared from bulk APIs because of manufacturer backorders or the compounded medication is not commercially available. They may exist prepared equally a single unit of measurement for an individual patient, or prepared as a batch in multiple units for an private patient or multiple patients.26
Compounders must follow current official USP <797> standards when compounding ophthalmic preparations; USP is expected to publish a revised <797> chapter in June 2019 with an effective date of December 2019, and a revised affiliate <800> will have outcome with the new <797>.24 These standards include the requirements for compounding personnel; environment and environmental monitoring; equipment; categories of sterile preparations; quality assurance; terminate-production testing; assigning BUDs; packaging, handling, and transport of compounded sterile preparations; use and storage; redispensing; patient educational activity and monitoring; and adverse events reporting. Compounders should read, empathize, and implement this chapter of the U.s. Pharmacopeia before preparing compounded sterile medications. In add-on to the USP <797> standards, in that location are a few published guidelines that are specific for ophthalmic preparations.22,33,34
Compounding Practices for the Preparation of Ophthalmics
Dosage forms, such as ocular injections, are usually very concentrated due to the volume required. Mathematical calculations used to develop the formula should exist double-checked to minimize fault. Decimal errors during ophthalmics' preparation could significantly increase or decrease the required dose by several fold and cause injury to the middle.22
To improve accuracy in measuring sterile ingredients existence withdrawn from vials or numberless, use the smallest syringe necessary to measure out the desired book. For example, use a iii mL syringe to measure a 2.five mL quantity rather than a five mL syringe. The five mL syringe has 0.two mL increments on it, whereas the 3 mL syringe has 0.1 mL increments. When using liquid from a glass ampule in an ophthalmic, filter it through a 5-µm filter when removing liquid from the ampule to remove any particulate matter, such as glass shards from opening the ampule. Although non required, sterile powders for injection that have been reconstituted should as well be filtered, if possible, through a v-µm filter to remove particulate matter, such as a cadre from the vial stopper.34
Quality assurance is of import in ophthalmic dosage forms' preparation. Compounders must inspect all ophthalmic dosage forms visually for clarity and particulate thing. Suspensions must be easily suspended when shaken, with no caking on the bottom. Ointments must exist smooth, not grainy. The colour must exist equally expected for that particular formulation. The final volume and weight should be as calculated and not vary more than than 10% from the theoretical volume or weight. Finally, the compounder should check the pH to ensure that the solution is inside a range suitable for the agile ingredient(s) and the eye itself.22,26
Although a compounded ophthalmic preparation for an individual patient does not need to be tested, batch-prepared ophthalmic dosage forms for multiple patients must undergo a quarantine menstruation and cease-preparation testing. The USP <797> specifies that a batch of more than 25 units must be sterility tested. This is a minimum standard. Sterility testing any batch that affects multiple patients or has several doses for an individual patient is prudent and must exist done according to the USP <797> standard. Certain ophthalmic preparations tin can be easily contaminated and support fungal growth.36
The USP <797> standard does not crave endotoxin or potency testing for ophthalmic preparations; however, these tests may be appropriate for sure ophthalmic dosage forms. Typically, endotoxins are known for causing fever and other complications when injected into the bloodstream. There have been reported cases of ocular injections contaminated with endotoxins causing toxic anterior segment syndrome, which is an astute inflammation of the heart's anterior segment that usually occurs following cataract surgery. Potency testing should be done initially with the development of a new formula to ensure that the concentration of active medication and the process to prepare the dosage grade is accurate.24
Another consideration for compounding ophthalmic preparations is container choice. For preservative-free preparations, it would exist prudent to package the preparation in single-dose sterile bottles or syringes. For preparations that accept brusque BUDs when stored at room temperature or refrigerated, the compounder may need to package the training in single-dose containers and instruct the patient, caregiver, or practitioner to freeze them and merely thaw a ane-solar day or 2-day supply at whatever given fourth dimension to extend the batch's BUD.26
SUMMARY
Although most ophthalmic dosage forms are relatively easy to prepare, several factors demand to be considered when choosing to chemical compound them:
- Target location of the eye requiring handling
- Limerick of the ophthalmic dosage form
- Requirements for the grooming of ophthalmic dosage forms
The compounder must as well assess, co-ordinate to the USP <797> standards and hazardous compounding guidelines or local, state and federal regulations, whether the environment or equipment is suitable to safely compound sterile and/or hazardous ophthalmic preparations. Properly preparing compounded ophthalmic dosage forms is essential in providing good quality preparations to effectively care for a fragile organ, the eye.
For more than data on ophthalmic compounding, consult the review articles by Allen on nuts of sterile compounding37 and suspensions/ointments.38
REFERENCES
- Hopkins Thousand, Pearson R. Ophthalmic Drugs—Diagnostic and Therapeutic Uses. 5th ed. London, UK: Elsevier; 2007.
- Eye Wellness Topics. National Eye Institute, National Institutes of Health. July x, 2015. Accessed at https://nei.nih.gov/, June 21, 2016.
- Venkata Ratnam B, Madhavi S, Rajesh P. Ocular drug delivery: an update review. Int J Pharm Bio Sci. 2011;i(4):437-446.
- Gaudana R. Ananthula HK, Parenky A. Mitra AK. Ocular drug commitment. AAPSJ. 2010;12(3):348-360.
- Baranowski P, Karolewicz B, Gajda M, et al. Ophthalmic drug dosage forms: characterisation and research methods. Sci World J. 2014:861904.
- Hanna C, Fraunfelder FT, Cablevision M, et all. The effect of ophthalmic ointments on corneal wound healing. Am J Ophthal. 1973;76(2):193-200.
- Summers A. Treating burns caused by hydrofluoric acid. Emerg Nurse. 2011;19(3):12-15.
- Plister RR. Chemical corneal burns. Int Ophthalmol Clin. 1984;24(2):157-168.
- Accordino A, Chambers RA, Thompson BC. The stability of a topical solution of cocaine hydrochloride. Austral J Hosp Pharm. 1996;26(6):629-633.
- Kallio H, Paloheimo M, Maunuksela Due east-50. Hyaluronidase as an adjuvant in bupivacaine-lidocaine mixture for retrobulbar/perbulbar cake. Anesth Analg. 2000;91(four):934-937.
- Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine center drops in vernal kertoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89(three):298-303.
- Wang Y, Ogawa Y, Dogru Thousand, et al.Ocular surface and tear functions afterwards topical cyclosporine handling in dry heart patients with chronic graft-versus-host illness. Os Marrow Transplant. 2008;41(3):293-302.
- Restasis [package insert]. Irvine, CA: Allergan;2013.
- McElhiney LF. Developing an erythromycin ophthalmic ointment – putting the puzzle pieces together. Int J Pharm Compound. 2010;fourteen(4):270-274.
- Allen LV Jr. Rose bengal 1% ophthalmic solution. Int J Pharm Compound. 1998;ii(3):231.
- MillodotM. Dictionary of Optometry and Visual Science. seventh ed. Edinburgh: Elsevier-Butterworth-Heinemann; 2009.
- Pickrell A, Harris A, Ngo Southward, et al. Commitment of intraocular triamcinolone acetate in the treatment of macular edema. Pharmaceutics.2012;four(1):230-242.
- Canavan KS, Dark A, Garrioch MA. Sub-Tenon's administration of local anaesthetics: a review of technique. Br J Anaesth.2003;90(six):787-793.
- Goldman DA. Intracameral therapy: the next stride in management of ocular disease? September xix, 2008. Accessed at http://www.ophthalmologyweb.com/Featured-Articles/20009-Intracameral-therapy-The-next-step-in-direction-of-ocular-disease/, June 22, 2016.
- Myers L, Almeida D, Abramoff MD. Intravitreal injection technique: a primer for ophthalmology residents and fellows. Jan vi, 2015. Accessed at world wide web.eyerounds.org/tutorials/intravitreal-injection/, June 22, 2016.
- Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29(7):875-912.
- McElhiney LF. Compounding Guide for Ophthalmic Preparations. Washington, DC: American Pharmacists Association; 2013.
- Kibbe AH. Handbook of Pharmaceutical Excipients. third ed. Washington, DC: American Pharmaceutical Clan; 2000.
- United States Pharmacopeial Convention. USP General Chapter <797> Pharmaceutical Compounding—Sterile Preparations. In: United States Pharmacopeia 38/National Formulary 33. Rockville, Doc: United States Pharmacopeial Convention; 2015.
- Trissel LA. Trissel's Stability of Compounded Preparations. 4th ed. Washington, DC: American Pharmacists Association; 2009.
- Allen Loyd V Jr. Chapter 21: Ophthalmic, Otic, and Nasal Preparations. In: The Art, Science, and Technology of Pharmaceutical Compounding. 4th edition. Washington, DC: American Pharmacists Clan; 2012:307-330.
- Chedru-Legros V, fines-Guyon Grand, Cherel A, et al. In vitro stability of fortified ophthalmic antibiotics stored at -20°C for 6 months. Cornea. 2010;29(7):807-811.
- United States Pharmacopeial Convention. USP monographs: bland lubricating ophthalmic ointment. In: U.S. Pharmacopeia 34/National Formulary 29. Rockville, Doctor: United States Phamacopeial Convention; 2011.
- U.S. Food and Drug Assistants. FDA alerts health intendance professionals of infection risk from repackaged Avastin intravitreal injections. Accessed at http://web.archive.org/spider web/20110901180651/http://world wide web.fda.gov/Drugs/DrugSafety/ucm270296.htm. June 22, 2018.
- Associated Press. Eye injuries linked to contaminated drug. New York Times. November ten, 1990. Accessed at http://world wide web.nytimes.com/1990/xi/10/us/heart-injuries-linked-to-contaminated-drug.html, June 22, 2016.
- Centers for Disease Control and Prevention. Outbreaks of postoperative bacterial endophthalmitis caused by intrinsically contaminated ophthalmic solutions-Thailand, 1992, and Canada, 1993. MMWR Morb Mortal Wkly Rep. 1996;45(23):491-494.
- United States Pharmacopeial Convention. USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings. December 1, 2014. Accessed at http://www.usp.org/usp-nf/notices/general-chapter-hazardous-drugs-handling-healthcare-settings, June 22, 2016.
- ASHP technical assistance bulletin on pharmacy-prepared ophthalmic products. Am J Hosp Pharm.1993;l(vii):1462-1463.
- Reynolds LA, Closson RG. Ad-lib Ophthalmic Preparations. Vancouver, WA: Applied Therapeutics; 1993.
- U.S. Food and Drug Assistants. Recall of brilliant bluish Yard urgent product recall—firsthand action required. March 9, 2012. Accessed at http://web.archive.org/web/20120318172103/http://world wide web.fda.gov:80/Condom/Recalls/ucm296326.htm, June 27, 2018.
- American Lodge of Cataract and Refractive Surgery, American Order of Ophthalmic Registered Nurses. Recommended practices for cleaning and sterilizing intraocular surgical instruments. J Cataract Refract Surg. 2007;33:1095-1100.
- Allen LV Jr. Basics of sterile compounding: ophthalmic preparations, role 1: ophthalmic solutions. Int J Pharm Compd. 2016(Sep-Oct);20(5):399-404.
- Allen LV Jr. Basics of sterile compounding: ophthalmic preparations, part ii: suspensions and ointments. Int J Pharm Compd. 2016(November-Dec);xx(6):495-500.
Back to Peak
« Render to Activity
Source: https://www.powerpak.com/course/print/113227
0 Response to "The Art Science and Technology of Pharmaceutical Compounding 3rd Edition"
Post a Comment